Battery applied sciences that may reliably function at very low temperatures may very well be extremely beneficial for a variety of purposes. These batteries might, as an illustration, energy units, autos, and robotic methods in outer area, deep beneath the ocean, and in different excessive environments.
To soundly and successfully function in these environments, batteries ought to have parts that don’t freeze or adversely react to important drops in temperature. One proposed answer is the design of rechargeable aqueous batteries containing so-called anti-freezing electrolytes.
Researchers on the Chinese language Academy of Sciences and different institutes in China lately devised a brand new technique to design anti-freezing electrolytes for aqueous batteries. This technique, outlined in a paper revealed in Nature Power, focuses on two particular temperature-related elements, which have to this point not been the first focus of anti-freezing electrolyte design.
“Designing anti-freezing electrolytes through choosing suitable H2O–solute systems is crucial for low-temperature aqueous batteries (LTABs),” Liwei Jiang, Shuai Han, and their colleagues wrote of their paper.
“However, the lack of an effective guideline for choosing H2O–solute systems based on decisive temperature-limiting factors hinders the development of LTABs. Here we identified two decisive factors: thermodynamic eutectic temperature (Te) and kinetic glass-transition temperature (Tg), with Tg being applicable for LTABs only when H2O–solute systems have strong super-cooling ability.”
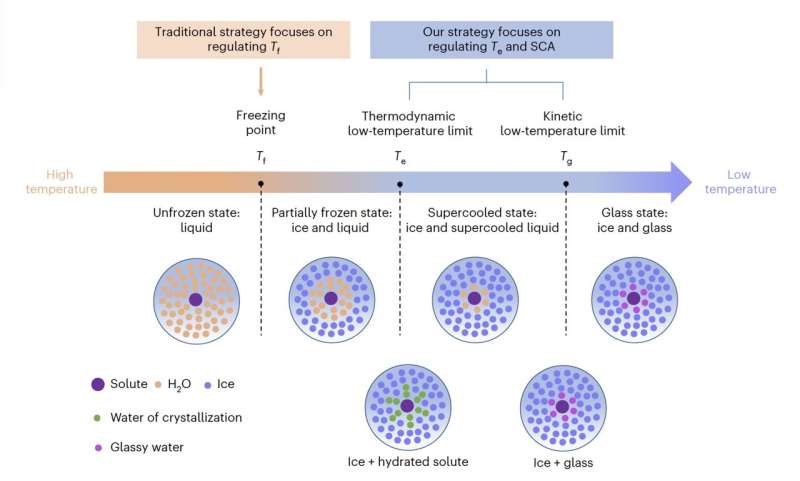
Most earlier works geared toward designing anti-freezing electrolytes centered on regulating the so-called freezing level (Tf) which is actually the precise level at which a liquid freezes and turns into strong. This may very well be achieved utilizing a wide range of totally different approaches.
Nonetheless, Tf won’t be essentially the most essential issue limiting the operation of batteries at low temperatures. Actually, some electrolytes permit batteries to function at low temperatures even when partially frozen, thus beneath their Tf level.
Of their paper, subsequently, Jiang, Han and their colleagues deal with two different temperature-related elements, specifically Te and Tg, The primary of those is the bottom temperature at which a liquid answer stays steady at a given strain, whereas the latter is the temperature at which molecular mobility begins to happen and beneath which this mobility turns into frozen, prompting a transition to a inflexible and glassy section.
“We proposed a general strategy wherein low-Te and strong-super-cooling ability electrolytes can be realized by creating multiple-solute systems via introducing assisted salts with high ionic-potential cations (for example, Al3+, Ca2+) or cosolvents with high donor numbers (for example, ethylene glycol),” the researchers wrote.
“As a demonstration in Na-based systems, we designed electrolytes with ultralow Te (−53.5 to −72.6 oC) and Tg (−86.1 to −117.1 oC), showcasing battery performances including 80 Wh kg−1 and 5,000 cycles at 25 oC, and 12.5 Wh kg−1 at −85 oC.”
Of their paper, the researchers proved {that a} detailed guideline that can be utilized to design anti-freezing electrolytes for aqueous batteries meant to function at low temperatures. Sooner or later, this guideline might show helpful to different analysis teams, doubtlessly contributing to the event of higher performing battery options for applied sciences designed to be deployed in area, within the deep sea and in different settings characterised by extraordinarily low temperatures.
Extra data:
Liwei Jiang et al, Rational design of anti-freezing electrolytes for terribly low-temperature aqueous batteries, Nature Power (2024). DOI: 10.1038/s41560-024-01527-5
© 2024 Science X Community
Quotation:
A technique to design anti-freezing electrolytes for batteries that may function in extraordinarily chilly environments (2024, Might 31)
retrieved 31 Might 2024
from https://techxplore.com/information/2024-05-strategy-anti-electrolytes-batteries-extremely.html
This doc is topic to copyright. Aside from any truthful dealing for the aim of personal research or analysis, no
half could also be reproduced with out the written permission. The content material is supplied for data functions solely.

