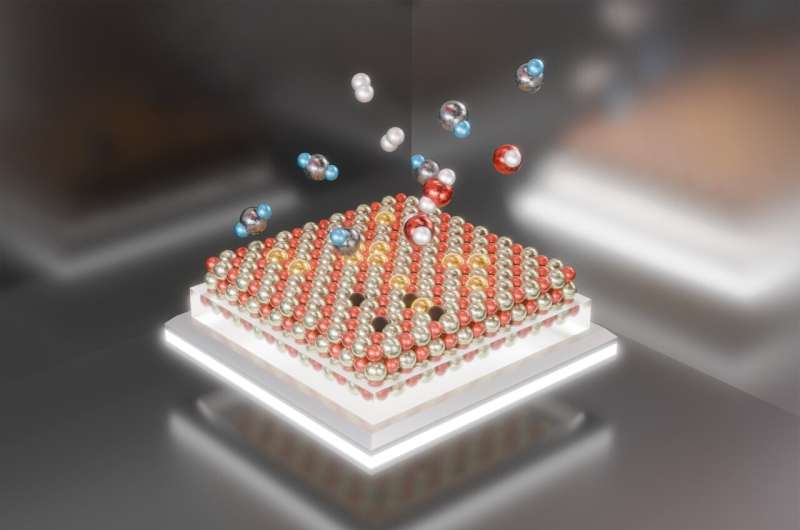
Chemists on the U.S. Division of Power’s (DOE) Brookhaven Nationwide Laboratory, Stony Brook College (SBU), and their collaborators have uncovered new particulars of the reversible meeting and disassembly of a platinum catalyst. The brand new understanding might supply clues to the catalyst’s stability and recyclability.
The work, described in a paper simply revealed within the journal Nanoscale, reveals how single platinum atoms on a cerium oxide help mixture below response circumstances to type energetic catalytic nanoparticles—after which, surprisingly, fragment as soon as the response is stopped.
Fragmentation might sound shattering, however the scientists say it might be a plus.
“Such reversible fragmentation of a platinum nanocatalyst on cerium oxide could be potentially useful for controlling the catalyst’s long-term stability,” stated Anatoly Frenkel, a chemist at Brookhaven Lab and professor at SBU who led the analysis.
When the platinum atoms return to their beginning positions, they can be utilized once more to remake energetic catalytic particles. Plus, the post-reaction fragmentation makes these energetic particles a lot much less more likely to fuse collectively irreversibly, which is a typical mechanism that in the end deactivates many nanoparticle catalysts.
“Part of the definition of a catalyst is that it helps disassemble and reassemble reacting molecules to form new products,” Frenkel famous. “But it was shocking to see a catalyst that also assembles and disassembles itself in the process.”
Meeting/disassembly
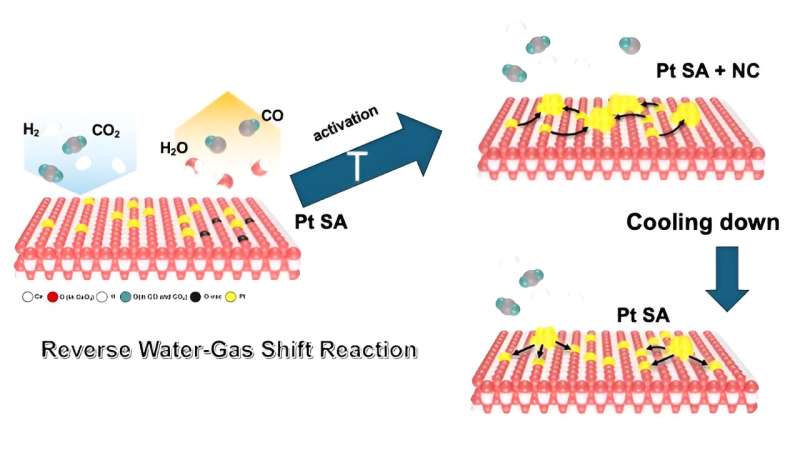
The paper describes how the scientists noticed the nanoparticles forming as single platinum atoms aggregated on the cerium oxide floor at 572 levels Fahrenheit (300 levels Celsius)—the temperature of the response they had been learning.
“After the reaction, we expected that these nanoparticles would stabilize once back at room temperature in whatever particle size they reached when they were activated,” Frenkel stated. “But what we observed was a reverse process. The particles began fragmenting into single atoms again.”
The staff had a speculation to elucidate what they had been seeing, which was confirmed by thermodynamic calculations carried out by colleagues at Chungnam Nationwide College in Korea. Carbon monoxide, one of many merchandise of the response—typically thought of a “poison” for catalysts—was actively tearing the nanoparticles aside.
“Carbon monoxide molecules have a very strong repulsive interaction when they are next to each other,” Frenkel defined. In the course of the “reverse water gas shift” response, which converts carbon dioxide (CO2) and hydrogen (H2) into carbon monoxide (CO) and water (H2O) at excessive temperatures, the CO usually leaves the catalyst floor as a gasoline. However as soon as the warmth is turned off, the CO molecules bind strongly to the platinum atoms of the catalyst. This brings the CO molecules nearer to one another because the system cools down and their numbers rise.
“That is a perfect storm,” stated Frenkel.
“When the CO molecules find themselves very close together on the surface of the nanoparticles, they repel. And, when they repel, because they are strongly bound to the platinum atoms, they sort of pull the least-tightly bound platinum atoms from the perimeter of the nanoparticle and drag them onto cerium oxide support,” Frenkel stated.
Multimodal imaging
The scientists used a mixture of atomic-level spectroscopic and imaging strategies to make these observations.
One method used vibrant X-rays on the Fast X-ray Absorption and Scattering beamline of the Nationwide Synchrotron Gentle Supply-II (NSLS-II) to provide a spectrum of the power absorbed by the atoms that make up the catalyst. The scientists used this system to check the catalyst at completely different temperatures and phases of the response. These X-ray absorption spectra are strongly influenced by the digital states of the atoms and can be utilized to decipher which atoms are close by.
“This technique can tell us that the platinum atoms have oxygen neighbors from the cerium oxide particles of the catalyst support, carbon monoxide neighbors from the reaction products, or other metal neighbors—more platinum atoms,” Frenkel stated. But it surely “lumps together information from many platinum atoms and only gives average information,” he famous.
“It can’t tell us whether all platinum atoms have the same environment or whether we have different groups of atoms—some dispersed on the support and some within the nanoparticles. We needed additional tools to unravel the possibilities,” he stated.
Infrared spectroscopy, carried out in Frenkel’s Construction and Dynamics of Utilized Nanomaterials (SDAN) laboratory within the Brookhaven Lab Chemistry Division, revealed the presence of two distinct teams —single atoms with no steel neighbors and nanoparticles made solely of platinum. The scientists used the method to trace the relative abundance of every group because the response progressed.
“This technique tells us how molecules such as CO interact with our platinum atoms. Do they show features of single atoms only or nanoparticles only or both?” Frenkel stated. “During the cooling down after the reaction, we observed that CO was interacting with single atoms again.”
Electron microscopy, carried out by Lihua Zhang of Brookhaven’s Heart for Purposeful Nanomaterials (CFN), produced nanoscale photos of each species—single atoms and nanoparticles. These photos present that, at room temperature earlier than the catalyst is activated, there are not any nanoparticles, and after the response, “we saw both nanoparticles and single atoms,” Frenkel stated.
“These techniques together tell us that, once the reaction stops and the temperature drops, the nanoparticles have started to fragment into single atoms,” Frenkel stated. “Each measurement independently would not have given us enough data to understand what we are dealing with. We couldn’t have done this work without our collaborators at NSLS-II and CFN and without the capabilities at these DOE Office of Science user facilities.”
Change and dysfunction
Understanding these variations at phases of the response is essential to understanding how the catalyst works, Frenkel stated.
“In our experiment, we deliberately went from one extreme to the other. We went from only single atoms to only nanoparticles. In the process, we had them coexist at different fractions so we could systematically investigate how the catalytic activity changes, how the structure changes,” he stated.
Frenkel famous that the nanoparticles do not assemble completely. They’ve extra defects—irregular atomic websites—in comparison with nanoparticles synthesized by generally used strategies. These defects may develop into one other function that improves catalytic efficiency.
That is as a result of dysfunction, or pressure, can contribute to the alignment of the digital ranges of chemical reactants and steel atoms within the catalyst to allow them to work together extra simply, he defined.
“People try to design catalysts with these types of imperfections deliberately; our method incorporates strain naturally,” he stated.
As well as, on account of these comparatively disordered constructions, nanoparticles assembled from single atoms may not be as tightly certain as an ideal array of atoms can be. That might make it simpler for them to disassemble for reuse when the response turns off.
Extra data:
Haodong Wang et al, Unravelling the origin of reaction-driven aggregation and fragmentation of atomically dispersed Pt catalyst on ceria help, Nanoscale (2024). DOI: 10.1039/D4NR01396D
Supplied by
Brookhaven Nationwide Laboratory
Quotation:
Examine reveals reversible meeting of platinum catalyst (2024, June 3)
retrieved 3 June 2024
from https://phys.org/information/2024-06-reveals-reversible-platinum-catalyst.html
This doc is topic to copyright. Other than any truthful dealing for the aim of personal examine or analysis, no
half could also be reproduced with out the written permission. The content material is offered for data functions solely.

