The design of battery applied sciences with more and more longer lifespans may assist to fulfill the rising wants of the electronics and automotive trade. Lithium (Li) batteries are probably the most broadly used rechargeable batteries worldwide. Thus, devising methods that may enhance their longevity could possibly be far simpler than designing completely new batteries.
Li-metal batteries, batteries with a Li steel anode, are among the many most promising next-generation battery designs. Nonetheless, the reactivity of Li steel has to date significantly restricted their biking stability, by impairing the formation of secure solid-electrolyte interphases (SEIs), finally leading to shorter battery lifespans.
Researchers on the Pennsylvania State College, College of Illinois Chicago and Argonne Nationwide Laboratory have lately launched a brand new methodology to increase the longevity of Li-metal batteries. This methodology, launched in a paper printed in Nature Vitality, depends on the usage of a extremely fluorinated cyclic ether (3,3,4,4,5,5-hexafluorotetrahydropyran, HFTHP), which reveals a restricted reactivity to Li steel ions and may thus enhance the steadiness of shaped SEIs.
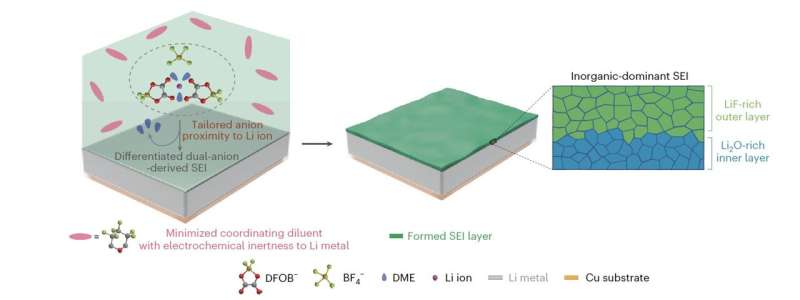
“Extending the lifespan of lithium (Li) batteries involves managing reactions at the Li anode and stabilizing the solid–electrolyte interphase (SEI) through strategic regulation of the electrolyte composition,” Guo-Xing Li, Volodymyr Koverga and their colleagues wrote of their paper. “We synthesized a fluorinated cyclic ether with minimized Li-ion coordination capability and enhanced electrochemical stability. We demonstrated its crucial role in manipulating the SEI formation process by differentiating the contribution of dual anions to the SEI layer.”
A vital distinction between the ether they synthesized and beforehand proposed linear fluorinated ethers, akin to BTFE and TTE, is that it reveals a minimized coordination to Li ions. This facilitates the formation of an inorganic-dominant bilayer SEI in metal-ion batteries, composed of a Li2O-rich internal layer and a LiF-rich outer layer.
This SEI was discovered to considerably increase the steadiness and reversibility of Li-metal anodes. The ensuing Li-metal battery cells have a remarkably lengthy biking life, improved self-discharge capabilities and high-temperature efficiency.
Li, Koverga and their colleagues used the ether to design a liquid electrolyte for Li-metal batteries composed of 1 M LiDFOB and 0.4 M LiBF4 in dimethoxyethane (DME)/HFTHP. They discovered that their design achieved distinctive outcomes, with a median CE of 99.5% for anode-free Cu||LiNi0.8Mn0.1Co0.1O2 (NMC811) cells and an prolonged lifespan.
“The developed electrolyte shows remarkable improvement in calendar life and cycling stability of Li (50 µm)||NMC811 (4 mAh cm−2) cells, maintaining 80% capacity after 568 and 218 cycles at room temperature and 60 °C, respectively,” the researchers wrote. “Furthermore, our 410 Wh kg−1 prototype pouch cells demonstrate 80% capacity retention for 470 cycles.”
The brand new technique launched by this analysis staff may quickly inform further research, finally facilitating the event of latest superior liquid electrolytes for high-density Li-metal batteries. Furthermore, their work contributes to the understanding of fluorinated ether diluents, highlighting their potential for the event of next-generation battery options.
Extra data:
Guo-Xing Li et al, Enhancing lithium-metal battery longevity by minimized coordinating diluent, Nature Vitality (2024). DOI: 10.1038/s41560-024-01519-5.
© 2024 Science X Community
Quotation:
Extending the lifespan of lithium-metal batteries utilizing a fluorinated ether diluent (2024, Might 24)
retrieved 24 Might 2024
from https://techxplore.com/information/2024-05-lifespan-lithium-metal-batteries-fluorinated.html
This doc is topic to copyright. Aside from any honest dealing for the aim of personal research or analysis, no
half could also be reproduced with out the written permission. The content material is offered for data functions solely.

