What if a standard ingredient, reasonably than scarce costly ones, was a key element in electrical automobile batteries? A collaboration co-led by an Oregon State College chemistry researcher is hoping to spark a inexperienced battery revolution by exhibiting that iron as an alternative of cobalt and nickel can be utilized as a cathode materials in lithium-ion batteries.
The findings, revealed in Science Advances, are necessary for a number of causes, Oregon State’s Xiulei “David” Ji notes.
“We’ve transformed the reactivity of iron metal, the cheapest metal commodity,” he stated. “Our electrode can offer a higher energy density than the state-of-the-art cathode materials in electric vehicles. And since we use iron, whose cost can be less than a dollar per kilogram—a small fraction of nickel and cobalt, which are indispensable in current high-energy lithium-ion batteries—the cost of our batteries is potentially much lower.”
At current, the cathode represents 50% of the price in making a lithium-ion battery cell, Ji stated. Past economics, iron-based cathodes would enable for larger security and sustainability, he added.
As an increasing number of lithium-ion batteries are manufactured to impress the transportation sector, world demand for nickel and cobalt has soared. Ji factors out that in a matter of a few a long time, predicted shortages in nickel and cobalt will put the brakes on battery manufacturing because it’s at present finished.
As well as, these parts’ vitality density is already being prolonged to its ceiling degree—if it had been pushed additional, oxygen launched throughout charging might trigger batteries to ignite—plus cobalt is poisonous, that means it will possibly contaminate ecosystems and water sources if it leaches out of landfills.
Put all of it collectively, Ji stated, and it is simple to know the worldwide quest for brand spanking new, extra sustainable battery chemistries.
A battery shops energy within the type of chemical vitality and thru reactions converts it to {the electrical} vitality wanted to energy automobiles in addition to cellphones, laptops and plenty of different gadgets and machines. There are a number of forms of batteries, however most of them work the identical fundamental means and include the identical fundamental elements.
A battery consists of two electrodes—the anode and cathode, usually made of various supplies—in addition to a separator and electrolyte, a chemical medium that permits for the movement {of electrical} cost. Throughout battery discharge, electrons movement from the anode into an exterior circuit after which gather on the cathode.
In a lithium-ion battery, as its title suggests, a cost is carried by way of lithium ions as they transfer by the electrolyte from the anode to the cathode throughout discharge, and again once more throughout recharging.
“Our iron-based cathode will not be limited by a shortage of resources,” stated Ji, explaining that iron, along with being the most typical ingredient on Earth as measured by mass, is the fourth-most considerable ingredient within the Earth’s crust. “We will not run out of iron ’til the sun turns into a red giant.”
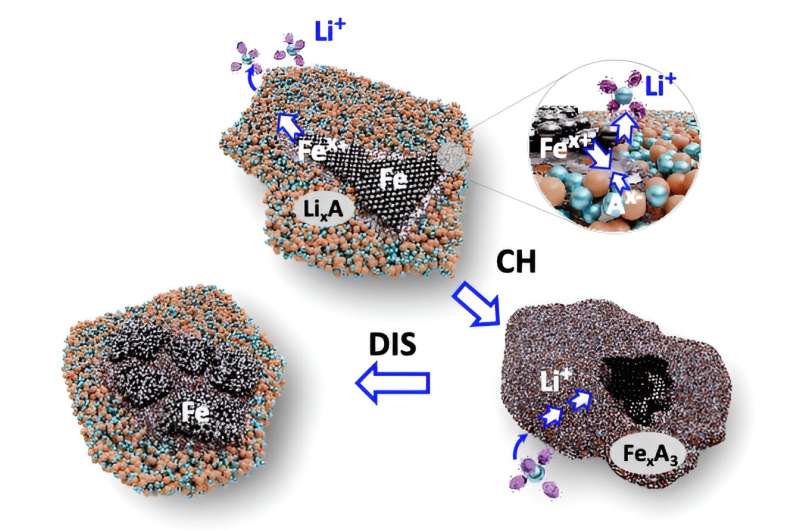
Ji and collaborators from a number of universities and nationwide laboratories elevated the reactivity of iron of their cathode by designing a chemical setting primarily based on a mix of fluorine and phosphate anions—ions which might be negatively charged.
The mix, totally blended as a stable resolution, permits for the reversible conversion—that means the battery may be recharged—of a tremendous combination of iron powder, lithium fluoride and lithium phosphate into iron salts.
“We’ve demonstrated that the materials design with anions can break the ceiling of energy density for batteries that are more sustainable and cost less,” Ji stated.
“We’re not using some more expensive salt in conjunction with iron—just those the battery industry has been using and then iron powder. To put this new cathode in applications, one needs to change nothing else—no new anodes, no new production lines, no new design of the battery. We are just replacing one thing, the cathode.”
Storage effectivity nonetheless must be improved, Ji stated. Proper now, not all the electrical energy put into the battery throughout charging is obtainable to be used upon discharge. When these enhancements are made, and Ji expects they are going to be, the consequence will likely be a battery that works a lot better than ones at present in use whereas costing much less and being greener.
“If there is investment in this technology, it shouldn’t take long for it to be commercially available,” Ji stated. “We need the visionaries of the industry to allocate resources to this emerging field. The world can have a cathode industry based on a metal that’s almost free compared to cobalt and nickel. And while you have to work really hard to recycle cobalt and nickel, you don’t even have to recycle iron—it just turns into rust if you let it go.”
The analysis was co-led by Tongchao Liu of Argonne Nationwide Laboratory and included Oregon State’s Mingliang Yu, Min Soo Jung and Sean Sandstrom.
Scientists from Vanderbilt College, Stanford College, the College of Maryland, Lawrence Berkeley Nationwide Laboratory and the SLAC Nationwide Accelerator Laboratory additionally contributed.
Extra data:
Mingliang Yu et al, Unlocking Iron Metallic as a Cathode for Sustainable Li-ion Batteries by an Anion Stable-Answer, Science Advances (2024). DOI: 10.1126/sciadv.adn4441. www.science.org/doi/10.1126/sciadv.adn4441
Oregon State College
Quotation:
Iron may very well be key to cheaper greener lithium-ion batteries, analysis finds (2024, Might 23)
retrieved 25 Might 2024
from https://techxplore.com/information/2024-05-iron-key-expensive-greener-lithium.html
This doc is topic to copyright. Aside from any honest dealing for the aim of personal examine or analysis, no
half could also be reproduced with out the written permission. The content material is supplied for data functions solely.

