Hydrogen is a promising chemical and vitality vector to decarbonize our society. Not like standard fuels, hydrogen utilization as a gasoline doesn’t generate carbon dioxide in return. Sadly, at the moment, a lot of the hydrogen that’s produced in our society comes from methane, a fossil gasoline. It does so in a course of (methane reforming) that results in substantial carbon dioxide emissions. Subsequently, the manufacturing of inexperienced hydrogen requires scalable alternate options to this course of.
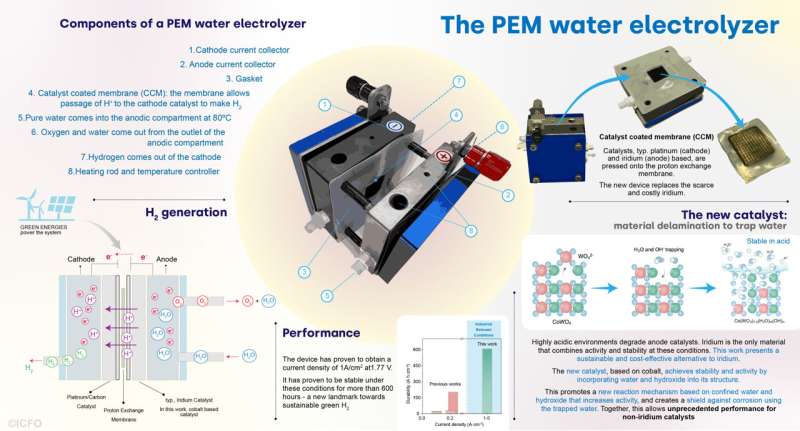
Water electrolysis gives a path to generate inexperienced hydrogen which could be powered by renewables and clear electrical energy. This course of wants cathode and anode catalysts to speed up the in any other case inefficient reactions of water splitting and recombination into hydrogen and oxygen, respectively. From its early discovery within the late 18th century, water electrolysis has matured into completely different applied sciences. One of the promising implementations of water electrolysis is the proton-exchange-membrane (PEM), which might produce inexperienced hydrogen combining excessive charges and excessive vitality effectivity.
Thus far, water electrolysis—and particularly PEM—has required catalysts based mostly on scarce, uncommon components, similar to platinum and iridium, amongst others. Just a few compounds mix the required exercise and stability within the harsh chemical surroundings imposed by this response. That is particularly difficult within the case of anode catalysts, which should function in extremely corrosive acidic environments—situations the place solely iridium oxides have proven secure operation on the required industrial situations. However iridium is without doubt one of the scarcest components on Earth.
Within the seek for attainable options, a staff of scientists has just lately taken an essential step to seek out alternate options to iridium catalysts. This multidisciplinary staff has managed to develop a novel approach to confer exercise and stability to an iridium-free catalyst by harnessing thus far unexplored properties of water. The brand new catalyst achieves—for the primary time—stability in PEM water electrolysis at industrial situations with out using iridium.
This breakthrough, printed in Science, has been carried out by ICFO researchers Ranit Ram, Dr. Lu Xia, Dr. Anku Guha, Dr. Viktoria Golovanova, Dr. Marinos Dimitropoulos, Aparna M. Das and Adrián Pinilla-Sánchez, and led by Professor at ICFO Dr. F. Pelayo García de Arquer; and consists of essential collaborations from the Institute of Chemical Analysis of Catalonia (ICIQ), The Catalan Institute of Science and Expertise (ICN2), French Nationwide Heart for Scientific Analysis (CNRS), Diamond Gentle Supply, and the Institute of Superior Supplies (INAM).
Coping with the acidity
Combining exercise and stability in a extremely acidic surroundings is difficult. Metals from the catalyst are likely to dissolve, as most supplies aren’t thermodynamically secure at low pH and utilized potential in a water surroundings. Iridium oxides mix exercise and stability in these harsh situations, and that’s the reason they’re the prevalent selection for anodes in proton-exchange water electrolysis.
The seek for alternate options to iridium just isn’t solely an essential utilized problem, however a basic one. Intense analysis within the seek for non-iridium catalysts has led to new insights into the response mechanisms and degradation, particularly with using probes that might examine the catalysts throughout operation mixed with computational fashions. These led to promising outcomes utilizing manganese and cobalt oxide-based supplies, and exploiting completely different constructions, composition, and dopants, to switch the physicochemical properties of the catalysts.
Whereas insightful, most of those research had been carried out in basic not-scalable reactors and working at softer situations which can be removed from the ultimate software, particularly when it comes to present density. Thus far, demonstrating exercise and stability with non-iridium catalysts in PEM reactors and at PEM-relevant working situations (excessive present density) had remained elusive.
To beat this, the ICFO, ICIQ, ICN2, CNRS, Diamond Gentle Supply and INAM researchers got here up with a brand new method within the design of non-iridium catalysts, attaining exercise and stability in acid media. Their technique, based mostly on cobalt (very plentiful and low cost), was fairly completely different from the standard paths.
“Conventional catalyst design typically focuses on changing the composition or the structure of the employed materials. Here, we took a different approach. We designed a new material that actively involves the ingredients of the reaction (water and its fragments) in its structure. We found that the incorporation of water and water fragments into the catalyst structure can be tailored to shield the catalyst in these challenging conditions, thus enabling stable operation at the high current densities that are relevant for industrial applications,” explains Professor at ICFO García de Arquer.
With their method, consisting of a delamination course of that exchanges a part of the fabric for water, the ensuing catalyst presents as a viable various to iridium-based catalysts.
A brand new method: The delamination course of
To acquire the catalyst, the staff appeared into a selected cobalt oxide: cobalt-tungsten oxide (CoWO4), or in brief CWO. On this beginning materials, they designed a delamination course of utilizing fundamental water options whereby tungsten oxides (WO42-) can be faraway from the lattice and exchanged by water (H2O) and hydroxyl (OH–) teams in a fundamental surroundings. This course of might be tuned to include completely different quantities of H2O and OH– into the catalyst, which might then be integrated onto the anode electrodes.
The staff mixed completely different photon-based spectroscopies to know this new class of fabric throughout operation. Utilizing infrared Raman spectroscopy and X-rays, amongst others, they had been capable of assess the presence of trapped water and hydroxyl teams, and to acquire insights on their function conferring exercise and stability for water splitting in acid.

“Being able to detect the trapped water was really challenging for us,” continues main co-author Dr. Anku Guha. “Using Raman spectroscopy and other light-based techniques we finally saw that there was water in the sample. But it was not ‘free’ water, it was confined water,” one thing that had a profound impression on efficiency.
From these insights, they began working carefully with collaborators and consultants in catalyst modeling.
“The modeling of activated materials is challenging as large structural rearrangements take place. In this case, the delamination employed in the activation treatment increases the number of active sites and changes the reaction mechanism, rendering the material more active. Understanding these materials requires a detailed mapping between experimental observations and simulations,” says Prof. Núria López from ICIQ.
Their calculations, led by co-author Dr. Hind Benzidi, had been essential to know how the delaminated supplies, shielded by water, weren’t solely thermodynamically protected towards dissolution in extremely acidic environments, but additionally lively.
However, how is that this attainable? Principally, the elimination of tungsten-oxide leaves a gap behind, precisely the place it was beforehand positioned. Right here is the place the “magic” occurs: Water and hydroxide, that are vastly current within the medium, spontaneously fill the hole. This in flip shields the pattern, because it renders the cobalt dissolution an unfavorable course of, successfully holding the catalyst parts collectively.
The staff assembled the delaminated catalyst right into a PEM reactor. The preliminary efficiency was actually outstanding, attaining larger exercise and stability than any prior work.
“We increased five times the current density, arriving at 1 A/cm2—a very challenging landmark in the field. But, the key is, that we also reached more than 600 hours of stability at such high density. So, we have reached the highest current density and also the highest stability for non-iridium catalysts,” shares main co-author Dr. Lu Xia.
“At the beginning of the project, we were intrigued about the potential role of water itself as the elephant in the room in water electrolysis,” explains Ranit Ram, first creator of the examine and instigator of the preliminary thought. “No one before had actively tailored water and interfacial water in this way.”
Ultimately, it turned out to be an actual game-changer.
Regardless that the steadiness time continues to be removed from the present industrial PEMs, this represents an enormous step in the direction of making them not depending on iridium or related components. Particularly, their work brings new insights for water electrolysis PEM design, because it highlights the potential to handle catalyst engineering from one other perspective; by actively exploiting the properties of water.
In the direction of the industrialization
The staff has seen such potential within the method that they’ve already utilized for a patent, with the intention of scaling it as much as business ranges of manufacturing. But, they’re conscious of the non-triviality of taking this step, as Prof. García de Arquer notes.
“Cobalt, being more abundant than iridium, is still a very troubling material considering from where it is obtained. That is why we are working on alternatives based on manganese, nickel and many other materials. We will go through the whole periodic table, if necessary. And we are going to explore and try with them this new strategy to design catalysts that we have reported in our study,” says Prof. García de Arquer.
Regardless of the brand new challenges that may for positive come up, the staff is satisfied of the potential of this delamination course of and they’re all decided to pursue this aim.
Ram, particularly, shares, “I have actually always wanted to advance renewable energies, because it will help us as a human community to fight against climate change. I believe our studies contributed one small step in the right direction.”
Extra data:
Ranit Ram et al, Water-hydroxide trapping in cobalt tungstate for proton alternate membrane water electrolysis, Science (2024). DOI: 10.1126/science.adk9849. www.science.org/doi/10.1126/science.adk9849
Quotation:
New catalyst unveils the hidden energy of water for inexperienced hydrogen technology (2024, June 20)
retrieved 25 June 2024
from https://techxplore.com/information/2024-06-catalyst-unveils-hidden-power-green.html
This doc is topic to copyright. Aside from any truthful dealing for the aim of personal examine or analysis, no
half could also be reproduced with out the written permission. The content material is supplied for data functions solely.

