Lithium-metal batteries may exhibit considerably larger vitality densities than lithium-ion batteries, that are the first battery know-how in the marketplace right now. But lithium-metal cells additionally sometimes have vital limitations, essentially the most notable of which is a brief lifespan.
Researchers at College of Science and Know-how of China and different institutes not too long ago launched a brand new electrolyte design that could possibly be used to develop extremely performing lithium-metal pouch cells with longer lifespans. This electrolyte, offered in a paper in Nature Power, has a singular nanometer-scale solvation construction, with pairs of ions densely packed collectively into compact ion-pair aggregates (CIPA).
“The primary objectives of our recent work are to markedly accelerate the practical applications of lithium-metal batteries and offer deep mechanistic understandings of this complicated system,” Prof. Shuhong Jiao, co-author of the paper, advised Tech Xplore.
“Li-metal batteries are the holy grail of the battery field and viewed as a promising next-generation battery technique, because they have ultra-high energy density, theoretically >500 Wh/kg. This is greater than 2-fold compared with today’s lithium-ion batteries dominating the battery market, which means that if we can replace lithium-ion batteries with lithium-metal batteries, the range of electric vehicles can be doubled per charge.”
Lithium-metal batteries launched to this point have a extremely restricted cycle life of roughly 50 cycles, which is considerably decrease than that of economic lithium-ion batteries, which might normally retain their good efficiency for roughly 1,000 cycles. The explanations behind this decrease lifespan are the expansion of lithium dendrites, the excessive reactivity of lithium-metal and high-voltage transition steel cathodes, which collectively immediate the fixed degradation of the electrolyte.
“Despite the extensive efforts of researchers around the world, the performance of lithium-metal batteries is still far from satisfactory (>500 Wh/kg, 1,000 cycles),” Prof. Jiao stated. “The primary cause is that the interfaces between electrolyte and electrodes (i.e., the anode-electrolyte interface and cathode-electrolyte interface) cannot be completely stabilized as in the case of lithium-ion batteries. Constant and severe electrolyte degradation still occurs during the battery operation.”
Roughly 5 years in the past, Prof. Jiao and her colleagues designed an electrolyte that may concurrently stabilize anode-electrolyte and cathode-electrolyte interfaces in lithium-metal battery cells, suppressing the electrolyte’s degradation. Their electrolyte design builds on early investigations of microscopic physicochemical processes inside lithium-metal batteries.
“An electrolyte is a key component of lithium-metal batteries, since it can tune the chemistry/structure of SEI and thus guide the plating behavior of lithium-metal, eventually dictating the battery performance,” Prof. Jiao defined.
“For practical application purposes, we tried to realize this by using cheap components. The countless works of other researchers in this field also inspired us a lot, as they introduced many new classes of electrolyte like highly concentrated electrolyte, localized high-concentration electrolyte, weak-solvating electrolyte and liquefied-gas electrolyte, etc.”
To hold out this current examine, Prof. Jiao and her analysis group teamed up with different groups that might carry out theoretical calculations and will characterize electrolytes at a microscopic scale. Their collaborative efforts in the end led to the design of a brand new class of electrolytes that may lengthen the lifespan of lithium-metal batteries.
The electrolytes they designed are manufactured from commercially accessible and inexpensive molecules. Their characterizing characteristic is their distinctive solvation construction.
“Solvation structure is a crucial inherent feature of an electrolyte, since it governs the interfacial behavior of electrolyte, like its interfacial reaction mechanism that controls the formation of SEI and thus the SEI chemistry and structure,” Prof. Jiao stated.
“The electrolyte solvation structure has been intensively tailored at the microscopic level in the scientific peer-reviewed literature so far, particularly the lithium ion’s first solvation shell, but the structural tuning beyond this scale, namely the second solvation shell and beyond, is largely overlooked.”
The current examine by Prof. Jiao and her colleagues has pioneered the tuning of an electrolyte’s solvation construction on the mesoscopic degree. Their distinctive design particularly focuses on the interplay between ion pairs underlying the formation of the electrolyte’s mixture construction.
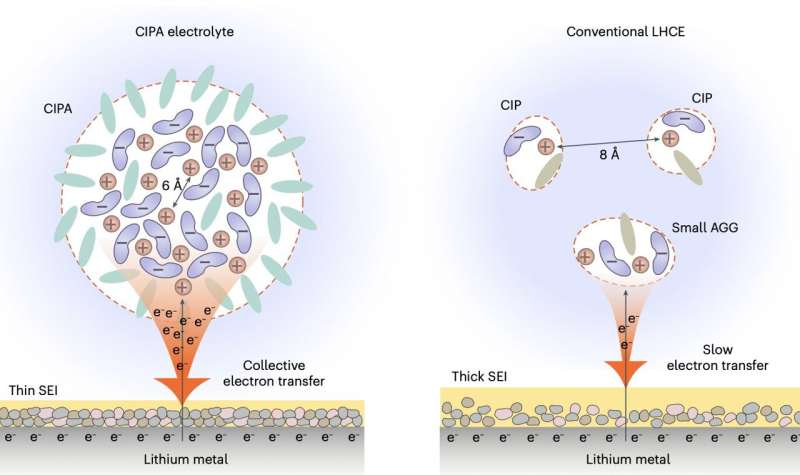
“Our electrolyte features large compact aggregates, which are formed by the dense packing of lithium-anion ion pairs with coordination bonding between each other, which we define as the ‘compact ion-pair aggregate (CIPA),” Prof. Jiao stated. “This marks stark contrast to the domination of small aggregates and separate ion pairs in the localized high-concentration electrolyte, a state-of-the-art electrolyte class with leading battery performance to-date, opening a new avenue for electrolyte design.”
Notably, the brand new electrolyte designed by this analysis workforce reveals a singular collective discount on the lithium-metal anode. Which means that clouds of anions within the CIPA construction are quickly lowered (i.e., decomposed) on the floor of the lithium, forming inorganic compounds akin to Li2O and LiF, in addition to a skinny and secure SEI, which in flip suppresses the fixed decomposition of the electrolyte.
“Thanks to the unique collective electron transfer behavior, our electrolyte forms a thin and conformal SEI with low organic content and rich in inorganic components with uniform distribution, which can promote the homogeneous lithium ion flux inside the SEI and dendrite-free lithium deposition,” Prof. Jiao stated. “This leads to a homogeneous and compact lithium deposition, which decreases the specific areas of lithium-metal anode to further suppress the electrolyte decomposition.”
Furthermore, the researchers’ newly designed electrolyte concurrently reveals good oxidative stability and suppresses the dissolution of transition steel components from the cathode, thus bettering the steadiness of the cathode interface. The stabilization of this interface, together with that of the lithium-electrolyte-interface, was discovered to translate to secure biking for a protracted variety of cycles.
“The mesoscopic solvation structure introduced in our paper leads to a new class of electrolytes, opening up a new avenue for electrolyte design of lithium-metal batteries,” Prof. Jiao stated.
To evaluate the potential of their newly designed electrolyte, the researchers used it to create a 500 Wh/kg lithium-metal pouch cell. In preliminary assessments, this cell was discovered to retain 91% of its vitality after working for 130 cycles. Sooner or later, this new electrolyte design could possibly be reproduced and examined by different researchers worldwide, to additional assess its potential for extending the lifetime of lithium-metal batteries.
“We are now planning to further prolong the cycle life of 500 Wh/kg lithium-metal pouch cells to more than 1,000 cycles,” Prof. Jiao added. “On the other hand, we are still exploring the new battery system to realize much higher energy density with a long lifespan, such as ≥ 600 Wh/kg with 100-200 cycles. All these fundamental scientific research studies are valuable to realize the deployment of lithium-metal batteries in many scenes.”
Extra data:
Yulin Jie et al, In the direction of long-life 500 Wh kg−1 lithium steel pouch cells through compact ion-pair mixture electrolytes, Nature Power (2024). DOI: 10.1038/s41560-024-01565-z
© 2024 Science X Community
Quotation:
Novel electrolyte design reveals promise for longer-lasting lithium-metal batteries (2024, August 18)
retrieved 18 August 2024
from https://techxplore.com/information/2024-08-electrolyte-longer-lithium-metal-batteries.html
This doc is topic to copyright. Other than any truthful dealing for the aim of personal examine or analysis, no
half could also be reproduced with out the written permission. The content material is supplied for data functions solely.

