Tri-layer could also be higher than bi-layer for manufacturing, enhancing the pace and capability of electrochemical and electrocatalytic units.
Three layers of graphene, in a twisted stack, profit from the same excessive conductivity to “magic angle” bilayer graphene however with simpler manufacturing—and sooner electron switch. The discovering may enhance nano electrochemical units or electrocatalysts to advance vitality storage or conversion.
Graphene—a single layer of carbon atoms organized in a hexagonal lattice—holds distinctive properties, together with excessive floor space, glorious electrical conductivity, mechanical power and adaptability, that make this 2D materials a robust candidate for rising the pace and capability of vitality storage.
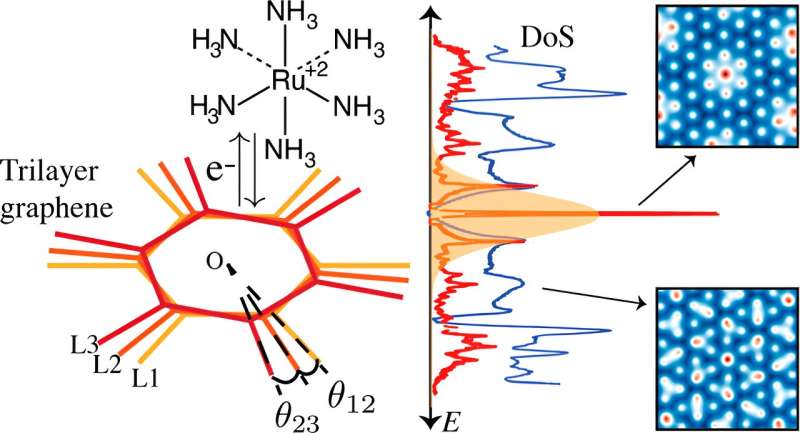
Twisting two sheets of graphene at a 1.1° angle, dubbed the “magic angle,” creates a “flat band” construction, that means the electrons throughout a variety of momentum values all have roughly the identical vitality. Due to this, there’s a large peak within the density of states, or the obtainable vitality ranges for electrons to occupy, on the vitality degree of the flat band which reinforces electrical conductivity.
Latest work experimentally confirmed these flat bands will be harnessed to extend the cost switch reactivity of twisted bilayer graphene when paired with an applicable redox couple—a paired set of chemical substances typically utilized in vitality storage to shuttle electrons between battery electrodes.
Including an extra layer of graphene to make twisted trilayer graphene yielded a sooner electron switch in comparison with bilayer graphene, in response to an electrochemical exercise mannequin in a latest examine by College of Michigan researchers.
“We have discovered highly flexible and enhanced charge transfer reactivity in twisted trilayer graphene, which is not restricted to specific twist angles or redox couples,” mentioned Venkat Viswanathan, an affiliate professor of aerospace engineering and corresponding writer of the examine printed within the Journal of the American Chemical Society.
Stacking three layers of graphene launched an extra twist angle, creating “incommensurate,” that means non-repeating patterns, at small-angle twists—not like bilayer graphene which varieties repeating patterns. Basically, when including a 3rd layer, the hexagonal lattices don’t completely align.
At room temperature, these non-repeating patterns have a wider vary of angles with excessive density of states away from the flat bands, rising electrical conductivity corresponding to these predicted on the magic angle.
“This discovery makes fabrication easier, avoiding the challenge of ensuring the precise twist angle that bilayer graphene requires,” mentioned Mohammad Babar, a doctoral pupil of mechanical and aerospace engineering and first writer of the examine.
As a subsequent step, the researchers plan to confirm these findings in experiments, and doubtlessly uncover even increased exercise in multi-layer twisted 2D supplies for a variety of electrochemical processes similar to redox reactions and electrocatalysis.
“Our work opens a new field of kinetics in 2D materials, capturing the electrochemical signatures of commensurate and incommensurate structures. We can now identify the optimal balance of charge-transfer reactivity in trilayer graphene for a given redox couple,” mentioned Babar.
Extra info:
Mohammad Babar et al, Twisto-Electrochemical Exercise Volcanoes in Trilayer Graphene, Journal of the American Chemical Society (2024). DOI: 10.1021/jacs.4c03464
Offered by
College of Michigan School of Engineering
Quotation:
Stacking three layers of graphene with a twist accelerates electrochemical reactions (2024, June 21)
retrieved 23 June 2024
from https://phys.org/information/2024-06-stacking-layers-graphene-electrochemical-reactions.html
This doc is topic to copyright. Other than any truthful dealing for the aim of personal examine or analysis, no
half could also be reproduced with out the written permission. The content material is supplied for info functions solely.

